
GJA1-20k, which contains the full Cx43 C-terminus but lacks transmembrane domains, is the most abundant and most common smaller isoform and is essential to full-length Cx43 trafficking ( Smyth and Shaw, 2013 Xiao et al., 2020) by recruiting cytoplasmic actin to organize trafficking pathways ( Basheer et al., 2017). However, GJA1 mRNA is subject to endogenous internal translation generating several N-terminal truncated isoforms ( Smyth and Shaw, 2013 Salat-Canela, 2014 Ul-Hussain et al., 2014). GJA1, which encodes Cx43, has a single coding exon and thus is not subject to splicing ( Smyth and Shaw, 2013). Little is known how the gap junction channel and organelle convey protection during preconditioning.
Mitochondrial fission how to#
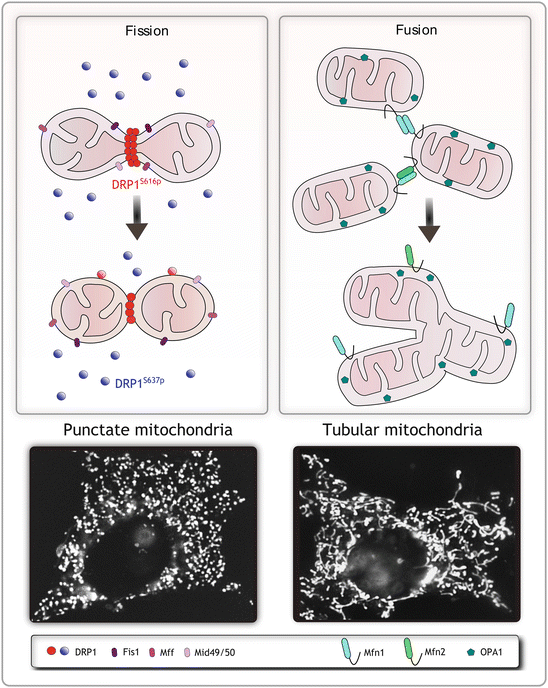
If we have a better understanding of conditions in which mitochondrial fission results from a protective mechanism, we will be closer to learning how to preserve mitochondrial fidelity in the setting of ischemic and reperfusion stress.īoth the gap junction protein Connexin43 (Cx43) and mitochondria are associated with preconditioning protection ( Garcia-Dorado et al., 2006 Basheer et al., 2018 Rodriguez-Sinovas et al., 2018). For instance, is mitochondrial fission a causative element of ROS generation, apoptosis, cellular senescence, and cell death ( Suen et al., 2008 Wang et al., 2017 Nishimura et al., 2018), or is fission actually protective, such as by promoting mitophagy which can promote survival ( Shirakabe et al., 2016 Burman et al., 2017)? The nuance in understanding the context of mitochondrial fission helps explain the complex relationship between mitochondrial morphology and function ( Nunnari and Suomalainen, 2012 Picard et al., 2013 Song et al., 2015). It is not clear if an overall shift to fission or fusion is sufficient to define mitochondrial fidelity, or whether a change in the fission-fusion equilibrium occurs secondary to multiple distinct pathways which could be either beneficial or harmful, depending on the pathway taken. Mitochondria undergo both fission and fusion in an adaptive equilibrium which directly affects cellular activity and response to stress ( Youle and van der Bliek, 2012 Friedman and Nunnari, 2014). Related to preconditioning is the perplexing dynamic regulation of mitochondria itself. Despite the large therapeutic potential of preconditioning for any organ such as heart, kidney, skeletal muscle, or brain subjected to anticipated ischemia, the mechanisms of preconditioning are not well understood, nor has an intervention been identified to successfully translate the phenomenon into clinical utility ( Heusch and Gersh, 2020). Interestingly, the phenomenon of ischemic preconditioning, first described 35 years ago ( Murry et al., 1986), is a potent yet ironic protection of organs from ischemia-induced damage achieved by preceding the full ischemic insult with shorter bouts of ischemia. Ischemia-Reperfusion (I/R) injury is known to induce excessive reactive oxygen species (ROS) in mitochondria, which results in cellular dysfunction and organ damage. The results indicate that stress responsive internally translated GJA1-20k stabilizes polymerized actin filaments to stimulate non-canonical mitochondrial fission which limits ischemic-reperfusion induced myocardial infarction. We find that GJA1-20k-induced smaller mitochondria have decreased reactive oxygen species (ROS) generation and, in hearts, provide potent protection against ischemia-reperfusion injury. Interestingly, GJA1-20k mediated fission is independent of canonical Dynamin-Related Protein 1 (DRP1). Mitochondrial fission events occur within about 45 s of GJA1-20k recruitment of actin. Here, using human cells and mice, we identify that GJA1-20k polymerizes actin around mitochondria which induces focal constriction sites. However, it is not known how GJA1-20k benefits mitochondria to provide this protection. Endogenous GJA1-20k protein is not membrane bound and has been found to increase in response to ischemic stress, localize to mitochondria, and mimic ischemic preconditioning protection in the heart. The Connexin43 gap junction gene GJA1 has one coding exon, but its mRNA undergoes internal translation to generate N-terminal truncated isoforms of Connexin43 with the predominant isoform being only 20 kDa in size (GJA1-20k).


 0 kommentar(er)
0 kommentar(er)
